Hayes S*, Kratzer U**, Pfeifer B***
* Department of Anesthesiology, Central Baptist Hospital, Lexington, Kentucky, USA
** Urology Clinic, Trostberg, Germany
*** Aeskulap Cancer Center, Brunnen, Switzerland
ABSTRACT
Purpose: ProstaSol™, a dietary supplement containing extracts from Saw Palmetto, Scutellaria, Ginseng, Skullcap, Reishi, Ginger, Stinging Nettle and Pygeum, as well as quercetin and a sitosterol mix, is becoming a popular alternative therapy among patients with hormone refractory prostate cancer. Anecdotal reports have claimed relief of metastatic pain, improvements in quality of life and reduction of the prostatic specific antigen (PSA) with this use of this product. Despite a lack of clinical data regarding safety and efficacy, an increasing number of patients in Europe and the United States are currently taking this herbal therapy.
Materials and Methods: A total of 96 patients with advanced metastatic prostate cancer (stage D3) were enrolled into a prospective clinical trial to evaluate possible toxic and beneficial effects of ProstaSol™. After failing hormone-ablative therapy and with established disease progression, all patients received supplemental treatment with ProstaSol™ (2880mg daily for the first month, 1800 mg per day for the second month, 1200 mg per day in the third months, and between 900 to 1200 mg from the fourth month on) for a total follow-up of nine months. Hormonal therapy was continued throughout the trial to avoid the known withdrawal effect of anti-androgen on PSA.
Results: Supplemental ProstaSol™ intake was associated with significant (p<0.05-0.01) improvements in quality of life measures, reductions in patients’ pain ratings (p<0.05-0.01), and a decline in PSA levels (p<0.01-0.001) without major side effects.
Conclusion: This study verifies anecdotal reports of the beneficial effects of ProstaSol™ as a comparable alternative to current management regimens in hormone refractory prostate cancer. No conclusions can be drawn regarding the long-term effects of this new herbal therapy.
INTRODUCTION
The incidence of prostate cancer has risen dramatically in recent years and will continue to rise as our population ages. In 1997, an estimated 335,000 new cases were reported in the United States alone, with approximately 42,000 deaths 1. Almost 50% of prostate cancer patients will eventually develop incurable disease 2, and of these patients 10-20% will have distant metastasis on initial presentation 1.
Current therapies for newly diagnosed prostate cancer include observation, prostatectomy, radiation therapy, cryotherapy, and/or hormonal therapy. Treatment choice is usually dictated by the patient’s PSA level, grade and stage of the tumor, and overall health. Once the disease has spread, there is no cure 3. Hormonal therapies achieved through orchiectomy or LHRH agonist +/- anti-androgen offer limited tumor suppression lasting from months to several years. Long- term responses are uncommon, and most prostate cancer patients enter a hormone-refractory state. Multiple salvage regimens for this condition have been proposed and are still under investigation, but none of the clinical trials so far has reported a significant patient benefit, let alone curative effects. Within these trials however, specific end points have recently changed from PSA partial response rates (greater than 50% decline in PSA) to the assessment of the patient’s quality of life. Due to a variety of unpleasant side effects and overall ineffectiveness of current medical therapies for hormone-refractory prostate cancer, many of these patients are seeking alternative means of therapy 4. A new herbal product known as ProstaSol™ is currently receiving increasing attention among this patient population. ProstaSol™ is available as a dietary supplement and consists of extracts from eight herbs: Saw Palmetto, Scutellaria, Ginseng, Skullcap, Reishi, Ginger, Stinging Nettle and Pygeum, as well as quercetin and a sitosterol mix. There are numerous anecdotal reports claiming that this compound decreases PSA values and relieves pain due to metastases without major side effects. An increasing number of patients throughout the United States and Europe are now taking ProstaSol™ as a supplement or alternative to their traditional therapy, despite the lack of clinical data regarding efficacy and safety.
Considering the growing use of ProstaSol™ among patients with hormone-refractory disease and the dismal prognosis of this patient population, we felt that a clinical evaluation of this herbal therapy regarding safety and efficacy was urgently needed. Therefore, we studied the effects of ProstaSol™ on pain condition, quality of life, and PSA levels, as well as the side effect profile of this product in patients with hormone refractory disease.
MATERIALS AND METHODS
From March through December 2001 a total of ninety six men with histologically proven prostatic adenocarcinoma, refractory to hormone-ablative therapy, gave informed consent to enter this prospective study. Urologists and anesthesiologists from Europe and the USA evaluated the effects of ProstaSol™ on pain, quality of life, and PSA levels. Each patient was diagnosed by transrectal, ultrasound guided prostatic biopsy. Hormone refractoriness was defined as three consecutive monthly increases in PSA levels. Disease progression was documented by magnetic resonance imaging (MRI), computerized tomography (CT), and bone scan (BS). All ninety six patients had hormone refractory (stage D3) disease at the beginning of the study. Patients were maintained on androgen-ablation therapy for the duration of the study, to avoid the known effect of anti-androgen withdrawal on PSA levels. All patients were asked to take supplemental ProstaSol™, three capsules, three times daily (total of 2880 mg/day) for one month, before a step-wise reduction of the dose followed (1800 mg per day for the second month, 1200 mg per day in the third months, and between 900 to 1200 mg from the fourth month on). The total follow-up was nine months. The manufacturer of ProstaSol™, medpro Holland B.V. of Scherpenzeel, The Netherlands, provided the study product.
Physical exams, blood chemistries, complete blood counts and PSA levels, as well as assessments of pain status, quality of life, and toxicity were completed for each patient before and after 4, 8, 12, 24 and 36 weeks of ProstaSol™ treatment. PSA of all patients was determined using the standard assay (Abbott Laboratories, Abbott Park, IL). Pain status was evaluated with a Visual Analog Scale from 0-10, with 0 = no pain and 10 = excruciating pain. Non-steroidal anti- inflammatory (NSAID) and narcotic drug intake was monitored during the entire study to further elucidate the effect of ProstaSol™ on pain control. Quality of life changes were determined using the FACT-P (Version 3) patient questionnaire 5, which contains several sets of specific questions regarding the physical, emotional, social and functional well-being. Each of these sets of questions was summarized by the patient in form of a numeric answer, with 0 – indicating no effect, and 10 – maximal effect on a particular aspect of the patient’s overall quality of life. Toxicity was evaluated using the Southwest Oncology Group (SWOG) Toxicity Criteria 6.
Data analysis was performed using Mann-Whitney Rank Sum and Equal Variance tests. Comparisons between control (pre-ProstaSol™) and 4, 8, 12, 24 and 36 weeks of ProstaSol™ intake were calculated for the following data: PSA, pain, quality of life and toxicity criteria. All data is presented as percent control (pre-ProstaSol™).
RESULTS
Table 1 presents some demographic information of the ninety six study patients. The mean age of the patients was 64.4 years (range 56-78 years), their mean body weight was at 82 kg (range 59 – 104 kg) at the begin of the study and reduced to 78 kg (range 58 – 96 kg) at the end of the study. All patients were pre-treated utilizing various standard therapies: 46 patients had a radical prostatectomy, 24 patients were radiated using brachytherapy and external beam radiation, respectively, 12 patients received complete androgen-ablation therapy, 10 had prior orchiectomy, and 4 patients had radiation therapy after prostatectomy. Sixty eight patients had concurrent bone and lymphatic metastases, 23 patients had only lymph node metastases, and another 5 Patients had no known metastases. Fifty six patients required narcotic and other pain medication for the treatment of their cancer related pain.
Table 1: Patients’ age, body weight and height data (mean and range)
| Mean | Range | |
| Age (years) | 64.4 | 56 – 78 |
| Body weight (kg) | 82.0 | 59 – 104 |
| Height (cm) | 178 | 169 – 200 |
Table 2 summarizes the ProstaSol™ effects on patients’ ratings for worst, average, least and current pain scores. Depicted are the mean values for each pain category assessed by the patient for a seven-day period prior to each follow-up visit. Pain scores for each pain category significantly (p<0.05-0.01) decreased during treatment with ProstaSol™. In addition, of the 56 patients who had to take narcotics or non-steroidal anti-inflammatory drugs (NSAID’s) for pain control prior to the study, 39 required about one third less of these analgesics at 12 weeks ProstaSol™ treatment.
| Table 2: ProstaSol effect on pain intensity | ||||||
| Pain – Categories | Weeks | |||||
| Baseline | 4 | 8 | 12 | 24 | 36 | |
| Worst Pain | 100 | *79 +/-7 | *71 +/-5 | *74+/-5 | **57 +/-6 | *55 +/-6 |
| Average Pain | 100 | *63 +/-4 | 63 +/-4 | *61 +/-6 | **48 +/-7 | 53 +/-8 |
| Present Pain | 100 | **55+/-4 | **60+/-5 | *70+/-5 | *55+/-5 | *50+/-8 |
| Least Pain | 100 | *46+/-9 | *44+/-8 | *51+/-11 | *45+/-6 | 48+/-7 |
Paired analogue data were analyzed using Mann Whitney Rank Sum Test and Equal Variance Test (* = p<0.05; ** = p<0.01). Data expressed as % change of pre-PC-SPES control values: mean +/- standard error.
ProstaSol™ had a positive effect on patient’s quality of life. In particular, evaluation in the area of physical and functional well being improved significant (p<0.05-0.001) during the intake of the herbal extract. Fifty eight of the 96 patients reported improvement of at least 30% in their overall daily functioning. The measure for social and emotional well being did not change significantly. This improvement in the quality of life measures was associated with minimal side effects in these patients. Forty three patients reported mild breast tenderness, 17 complaint of full stomach feeling and 6 patients had a short bout of diarrhea during the first weeks of intake. There were no significant changes in the blood parameter, other than PSA decline, and there was no case of venous thrombosis in these patients during the ProstaSol™ intake.
Figure 2 shows the effects of ProstaSol™ treatment on PSA levels. Depicted are the group means +/- standard error of the percent change compared to the pre-study control. A highly significant (p<0.01-0.001) decline in PSA levels was seen following ProstaSol™ therapy.
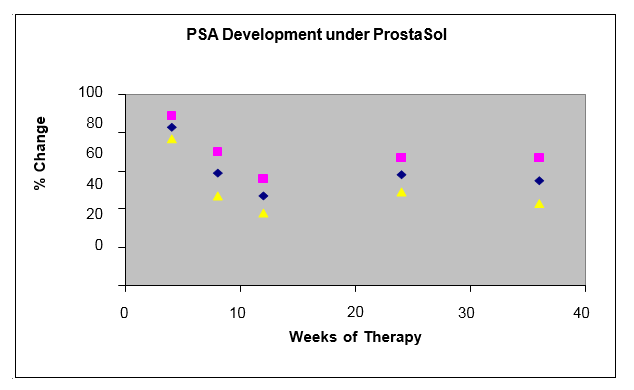
The mean PSA level prior to treatment was 87 ng / ml (range from 16-203 ng / ml). The intake of ProstaSol™ was associated with a significant (p<0.01-0.001) decline of the PSA levels. A mean PSA nadir of 41 ng / ml was reached by about 10 to 12 weeks into the treatment. Twelve patients reached their nadir at 8 weeks of therapy. In 65 of the 96 patients there was a >50% PSA decline when compared with the level prior to ProstaSol™ treatment. In 24 patients the PSA response lasted only 3 to 4 months, after which their PSA began to rise again.
DISCUSSION
Patients with hormone refractory prostate cancer are reported to have a median survival of six to twelve months 7. Current therapeutic regimens have only shown transient palliative benefit without increasing patient survival 7, and most of these palliative therapies have significant side effects. Patients with such dismal prognosis are prone to seek alternative treatments, including herbal remedies 4,16. Among such remedies, ProstaSol™ has gained some popularity due to anecdotal reports about its benefits for hormone refractory patients. The results of our study verify some of those claimed benefits.
The highly significant reduction in reported pain scores and analgesic drug intake in our patient group suggests a pain relieving effect of ProstaSol™. Since the product tested free of any analgesic drug admixture, the observed pain relief is interpreted as a direct effect of ProstaSol™. At least four components of ProstaSol™, pygeum, reishi (Ganoderma lucidum), ginger and panax pseudo-ginseng, are known to have anti-inflammatory and analgesic effects that could explain the greater than 30% reduction in analgesic drug use in the majority of our patients. In at least three patients, a decreased volume of metastatic disease also may have contributed to the reduced analgesic drug requirement, since their repeat bone scans and CT at the 24-week follow-up revealed a decline in the number of bone lesions, and reduced pelvic lymphadenopathy. The observed pain relief reported by our patients was associated with an overall improvement in their quality of life. Ability to better ambulate, more energy, and increased appetite were observed by about 60% of our patients, effects that may in part be due to the ginseng, pygeum and reishi components of ProstaSol™.
The finding that about two third of the hormone refractory patients responded with a greater than 50% reduction in their serum PSA levels indicates a high efficacy of ProstaSol™. This herbal product therefore can reduce the PSA production and/or release from prostate cells and it seems logical to assume that ProstaSol™ also slows down, or even inhibits cancer growth. There is a large body of evidence from experimental research showing a cancer-inhibitory effect of most ProstaSol™ components. Chan et al 8, for example, could demonstrate that extracts from Scutellaria contain the flavonoid baicalin, which can produce apoptosis in minute concentration in the DU145 prostate cancer cell model. Similar results were published by Hsieh und Wu 9 showing that scutellaria extracts could inhibit the growth of the prostate cancer cell line LnCaP by more than 60%, and thereby reduce PSA production. Stinging nettle also is known to cause growth inhibition in prostate cancer cells. Lichius et al 10 demonstrated this for a polysaccharide fraction of the stinging nettle root extracted with 20% methanol. This extract inhibited prostate cancer cells from lymph node metastases by greater than 50%. Ganoderma lucidum extracts cause anti-tumor effects through release of cytokines such as tumor necrosis factor (TNF-alpha) and interferon (INF-gamma) 11. Knowles et al 12 published that quercetin in a concentration of less than 100 micromole caused a complete growth inhibition of the hormone-resistant prostate cancer cell line, PC-3. Surh 13 reported anti-cancer activity for two ginger phenols, 6-gingerol and 6-paradol, and Liu et al 14 showed that Saponine and Ginsenoside (Rg-3), extracted from ginseng, caused pronounced inhibition of prostate cancer cell growth associated with a reduction in PSA and androgen-receptor expression. Ginseng extracts also produced classical apoptosis through inhibition of bcl-2 gene activity, and reduced the metastatic potential of prostate cancer cells. Finally, Iguchi and co-workers 15 described similar apoptotic effects of Serenoa repens extract, which was at least in part caused by the cytotoxic properties of myristoleic acid found in that extract.
The ProstaSol™ therapy was well tolerated by the patients of this study. The following side effects were reported: bloated feeling in 18 patients (19%), particularly with the higher doses at the beginning of the treatment, diarrhea in 11 patients (12%), occurring during the first weeks of treatment and increased sensibility and slight swelling of the breast nipples in 32 patients (33%). There were no reports of thrombosis or any changes in the blood parameters, other than PSA. PPOSTASOL therefore seems to have a very mild side effect profile.
How long the PSA decreasing effect of PPOSTASOL will last, can not be determined from this study. PSA was still decreased in 41 of the 96 patients at 8 months follow-up. Twenty four patients, however, showed a recurrent PSA increase starting already after 4 months of initial PSA decline. Lastly, it is unclear, whether the observed PSA effect seen in these patients was indeed evidence for a reduction in tumor load, since repeat CT and bone scan at study completion were not required. The improvement of the two bone scans and the CT of three patients mentioned above, however, warrant further studies of ProstaSol™ in the treatment of hormone refractory prostate cancer.
CONCLUSION
Most patients with advanced prostate cancer who are on hormonal therapy will eventually develop refractory disease. At this point, there is no curative therapy. Partial PSA responses have been demonstrated in some chemotherapeutic trials, but due to the cytotoxic effects of chemotherapeutic agents on healthy viable cells, unpleasant side effects are inevitable. With quality of life becoming a more important end point in therapy, many of these patients turn to non-toxic alternatives, including herbal remedies. ProstaSol™, a blend of eight herbal extracts and sitosterol, as well as quercetin, significantly reduces PSA and pain of metastatic disease, thereby improving patients’ quality of life without the detrimental side effects seen with other drug regimens. With no cure available at this time for this patient population, maintaining a good quality of life is a realistic therapeutic goal that can be achieved with the dietary supplement, ProstaSol™.
REFERENCES
- Parker, S.L., Tong, T., Bolden, S., Wingo, P.A.: Cancer statistics, 1997. CA Cancer J Clin 47: 5, 1997
- Klien, L.A.: Prostatic carcinoma. N Engl J Med 300: 824, 1979
- Waselenko, J.K., Dawson, N.A.: Management of Progressive Metastatic Prostate Cancer. Oncology 11: 1551, 1997
- Eisenberg, D.M., Kessler, R.C., Foster, C., Norlock, F.E., Calkins, D.R., Delbanco, T.L.: Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med 328: 246, 1993
- Esper, P., Mo, F., Chodak, G., Sinner, M., Cella, D., Pienta K.J.: Measuring Quality of Life in Men with prostate Cancer using Functional Assessment of Cancer Therapy – Prostate Instrument. Urology 50:920, 1997
- Green, S., Weiss, G.R.: Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Investigational New Drugs 10:239, 1992
- Waselenko, J.K., Dawson, N.A.: Management of Progressive Metastatic Prostate Cancer. Oncology 11: 1551, 1997
- Chan FL, Choi HL, Chen ZY, Chan PS, Huang Y.: Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett 160(2):219, 2000
- Hsieh TC, Wu JM. Mechanism of action of herbal supplement PC-SPES: Elucidation of effects of individual herbs of PC-SPES on proliferation and prostate specific gene expression in androgen- dependent LNCaP cells. Int J Oncol 20(3):583, 2002
- Lichius JJ, Lenz C, Lindemann P, Muller HH, Aumuller G, Konrad L.: Anti-proliferative effect of a polysaccharide fraction of a 20% methanolic extract of stinging nettle roots upon epithelial cells of the human prostate (LNCaP). Pharmazie 54(10):768, 1999
- Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS, Shiao MS, Ho CK.: The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer 70(6):699, 1997
- Knowles LM, Zigrossi DA, Tauber RA, Hightower C, Milner JA.: Flavonoids suppress androgen- independent human prostate tumor proliferation. Nutr Cancer 38(1):116, 2000
- Surh Y.: Molecular mechanisms of chemo-preventive effects of selected dietary and medicinal phenolic substances. Mutat Res 428(1-2):305,1999
- Liu WK, Xu SX, Che CT.: Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci 67(11):1297, 2000
15. Iguchi K, Okumura N, Usui S, Sajiki H, Hirota K, Hirano K.: Myristoleic acid, a cytotoxic component in the extract from Serenoa repens, induces apoptosis and necrosis in human prostatic LNCaP cells. Prostate 47(1):59, 2001
16. Risberg, T., Lund, E., Wist, E. Kaasa, S., Wilsgaard, T.: Cancer patients use of non-proven therapy: a 5-year follow-up study. J Clin Oncol 16:6, 1998
